Clinical Trial Sponsor
Clinical trial sponsors and the territorial scope of GDPR There is a compelling argument that the processing undertaken by the sponsor triggers the application of the GDPR under Article 32b even when the sponsor is located outside of the EEA because the sponsor is effectively monitoring the behavior of data subjects within the EEA. How feasible are they.
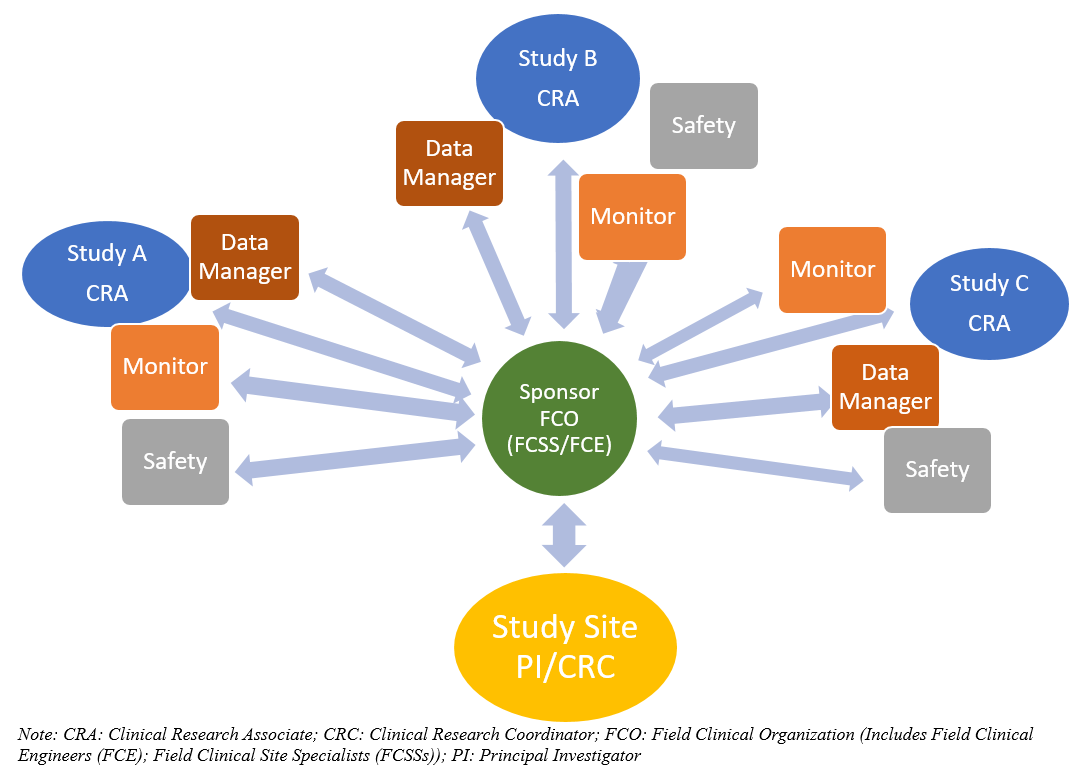 Sponsor Site Communication In Device Trials Evolution Of A Dedicated Field Clinical Organization Throughout Study Execution Acrp
Sponsor Site Communication In Device Trials Evolution Of A Dedicated Field Clinical Organization Throughout Study Execution Acrp
Ad Download your free virtual clinical trials resource bundle.

Clinical trial sponsor. Clinical trials can also be sponsored by individual Australian researchers eg. Specify the Clinical Trials andor Collaborators If you already have a list of clinical trials or collaboratorssponsors you would like to monitor for updates simply. The EU Clinical Trials Register website makes this.
A clinical trial sponsor is the company or organisation which conducts a clinical trial. A sponsor can transfer any or all of its trial-related duties to a contract research organization or CRO but the ultimate responsibility for the. This is usually done by the Sponsor in order to provide additional resource to the trial site or personnel with particular experience or skill to conduct specific procedures of the trial.
How feasible are they. A clinical trial DMC is a group of individuals with pertinent expertise that reviews on a regular basis accumulating data from one or more ongoing clinical trials. Are virtual clinical trials better.
Advanced analytics for identifying researchers and research sites with expertise in a given area utilize public and private information about. Hospitals area health services nongovernment organisations cooperative research groups. Novartis is a Swiss multi-national pharma company with headquarters in Basel Switzerland.
A sponsor-investigator on the other hand takes on the. Review and approval of the consent document is a responsibility that FDA assigns to the IRB with jurisdiction The regulations governing FDAregulated and federallyfunded research 21 CFR 50 56. The company employs over 118000.
Novant Health Clinical Research was established in 2001 and represents a unique model for conducting and managing research. Clinical Trial Sponsors 1. A clinical trial sponsor is the individual company institution or organization which takes responsibility for the initiation management andor financing of a clinical trial.
Clinical trials can be sponsored by organizations such as a pharmaceutical company Federal offices and agencies such as the National Institutes of Health or the US. Understand the roles and responsibilities of the sponsor involved in conducting a clinical trial. Find answers to these questions.
Department of Veterans. The sponsor is also responsible for ensuring that appropriate approvals are obtained prior to the commencement of the clinical trial that conditions of any approvals are adhered to during the course of the clinical trial and ensures that the ethics principles of research merit and integrity justice beneficence and respect are applied to the conduct of clinical trials. For Clinical Trials of Investigational Medicinal Products CTIMPs the term sponsor is defined in the Clinical Trial Regulations as.
Ad Download your free virtual clinical trials resource bundle. The sponsor of a clinical trial may in particular cases consider necessary to provide the investigational site with personnel to be involved directly in the conduct of the clinical trial. Clinical trial sponsors play an important role in determining the viability of a clinical study.
The sponsor should be a locally registered business entity registered with the Accounting and. Clinical Trial and Sponsor Monitoring Made Easy. The person who takes responsibility for the initiation management and financing or arranging the financing of that trial.
The sponsor is a company institution or organisation that takes responsibility for the initiation management andor financing of a clinical trial. Clinical trial management systems are often used by research sponsors or CROs to help plan and manage the operational aspects of a clinical trial particularly with respect to investigational sites. In the conduct of a clinical trial a sponsor is an individual institution company or organization for example a contract research organization that takes the responsibility to initiate manage or finance the clinical trial 1 but does not actually conduct the investigation.
Sponsors are responsible for overseeing the studys continued progress. It was created in 1996 following the merger of Ciba-Geigy and Sandoz. We have an integrated network of geographically dispersed physicians and research staff who offer extensive clinical research experience.
Let Clinical Trials Watch do all the work in three simple steps. Corporate Clinical Trial Sponsors of 2016. Find answers to these questions.
The DMC advises the sponsor. Medical practitioners or bodies and organisations eg. Trial sponsors ensure that a trial is.
Are virtual clinical trials better. Sponsors provide protocol related information to national competent authorities who then enter the information into a database called EudraCT. They oversee the overall study design facilitate funding sources and develop beneficial procedures to help guide the study towards a successful outcome.
 1 The Phases Of Clinical Trial Research Clinical Trials Pass Through A Download Scientific Diagram
1 The Phases Of Clinical Trial Research Clinical Trials Pass Through A Download Scientific Diagram
 When Why And How Sponsor Contract Research Organisation Cros And Research Sites Work Together Your Pharmacy Guide
When Why And How Sponsor Contract Research Organisation Cros And Research Sites Work Together Your Pharmacy Guide
![]() The Latest Clinical Trial Portal Alternative 2020
The Latest Clinical Trial Portal Alternative 2020
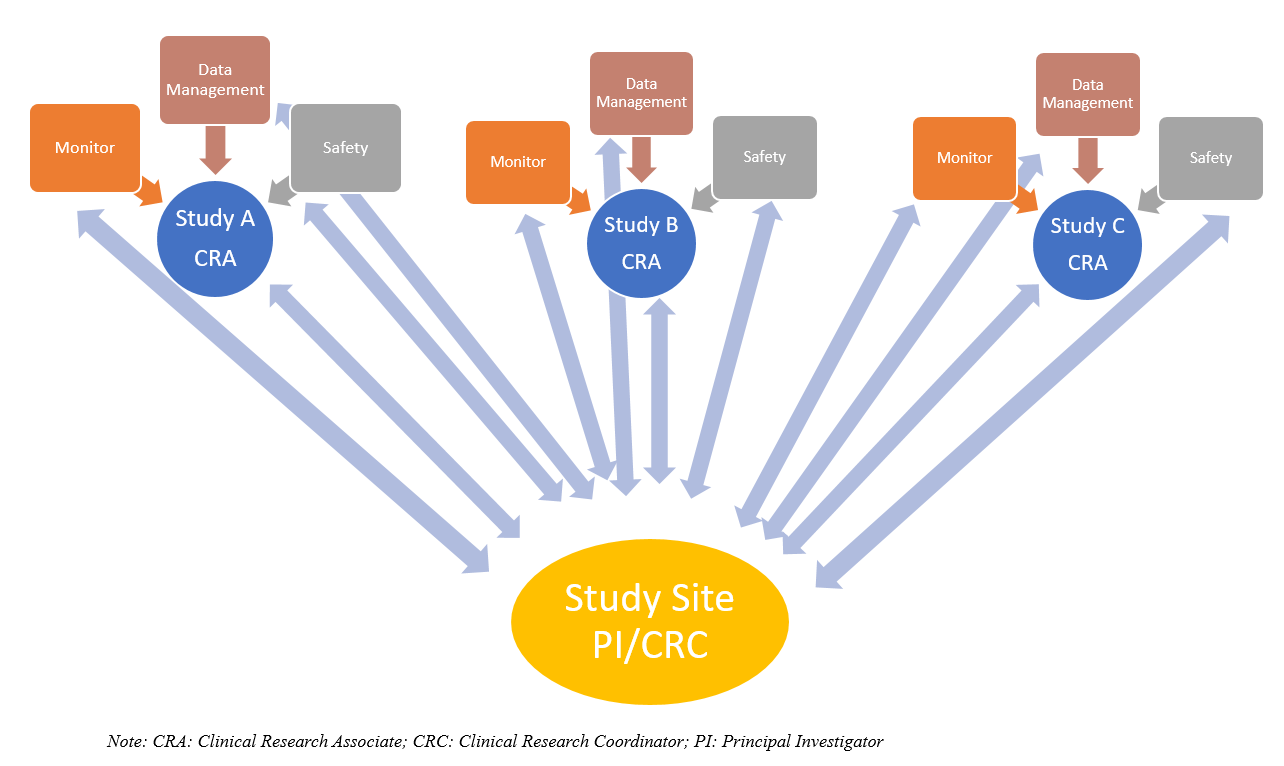 Sponsor Site Communication In Device Trials Evolution Of A Dedicated Field Clinical Organization Throughout Study Execution Acrp
Sponsor Site Communication In Device Trials Evolution Of A Dedicated Field Clinical Organization Throughout Study Execution Acrp
Quality Of Outsourced Trials Why Do Disconnects Still Exist Between Sponsors And Cros
![]() The Latest Clinical Trial Portal Alternative 2020
The Latest Clinical Trial Portal Alternative 2020
New Regulation On Clinical Trials In Spain Leon Research Cro Clinical Trials Spain Italy And Portugal
 Clinical Trial Players Youtube
Clinical Trial Players Youtube
 Roles And Responsibilities Of Sponsor In Conducting Clinical Trials A
Roles And Responsibilities Of Sponsor In Conducting Clinical Trials A
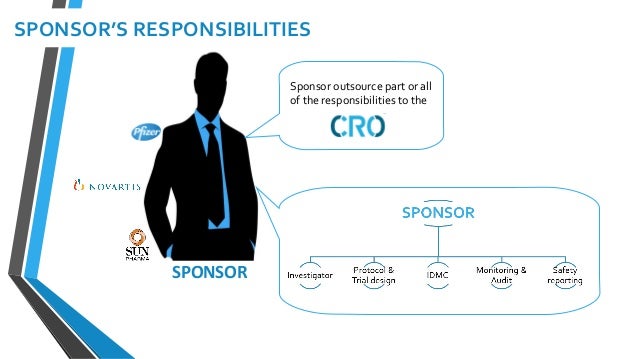 Key Stakeholders In Clinical Research Suchismita Banik
Key Stakeholders In Clinical Research Suchismita Banik
 Information For Sponsors Palmetto Clinical Research
Information For Sponsors Palmetto Clinical Research
 Responsibilities Of Sponsor Investigator And Monitor Ppt Video Online Download
Responsibilities Of Sponsor Investigator And Monitor Ppt Video Online Download
 Who Are Clinical Trial Sponsors
Who Are Clinical Trial Sponsors

Comments
Post a Comment